Just tuning in? Start with Respiration and Hemoglobin, or continue to Pulse Oximetry: Application
Once upon a time, the only way to measure SaO2 was to draw a sample of arterial blood and send it down to the lab for a rapid analysis of gaseous contents — an arterial blood gas (ABG), or something similar. This result is definitive, but it takes time, and in some patients by the time you get back your ABG, its results are already long outdated. The invention of a reliable, non-invasive, real-time (or nearly so) method of monitoring arterial oxygen saturation is one of the major advances in patient assessment from the past fifty years.
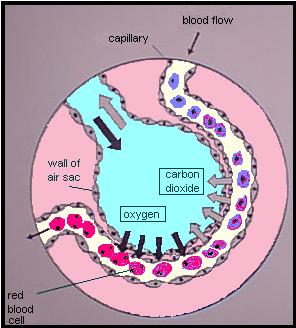
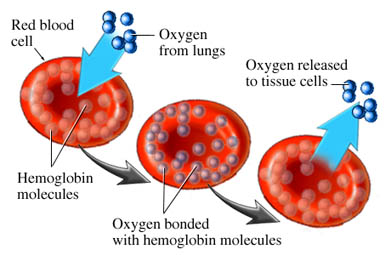
Oximetry relies on a simple principle: oxygenated blood looks different from deoxygenated blood. We all know this is true. If you cut yourself and bleed from an artery — oxygenated blood — it will appear bright red. Venous blood — deoxygenated — is much darker.
We can take advantage of this. We place a sensor over a piece of your body that is perfused with blood, yet thin enough to shine light through — a finger, a toe, maybe an earlobe. Two lights shine against one side, and two sensors detect this light from the other side. One light is of a wavelength (infared at around 800–1000nm) that is mainly absorbed by oxygenated blood; the other is of a wavelength (visible red at 600–750nm) that is mainly absorbed by deoxygenated blood. By comparing how much of each light reaches the other side, we can determine how much oxygenated vs. deoxygenated blood is present.
The big turning point in this technology came when “oximetry” turned into “pulse oximetry.” See, the trouble with this shining-light trick is that there are a lot of things between light and sensor other than arterial blood — skin, muscle, venous blood, fat, sweat, nail polish, and other things, and all of these might have differing opacity depending on the patient and the sensor location. But what we can do is monitor the amount of light absorbed during systole — while the heart is pumping blood — and monitor the amount absorbed during diastole — while the heart is relaxed — and compare them. The only difference between these values should be the difference caused by the pulsation of arterial blood (since your skin, muscle, venous blood, etc. are not changing between heartbeats), so if we subtract the two, the result should be an absorption reading from SaO2 only. Cool!
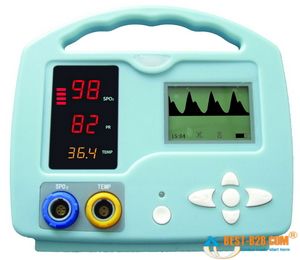
Most oximeters give you a few different pieces of information when they’re applied. The most important is the SaO2, a percentage between 0% and 100% describing how saturated the hemoglobin are with oxygen. (Typically, in most cases we refer to this number as SpO2, which is simply SaO2 as determined by pulse oximetry. This can be helpful by reminding us that oximeters aren’t perfect, and aren’t necessarily giving us a direct look at the blood contents, but for most purposes they are interchangeable terms.) But due to the pulse detection we just described, most oximeters will also display a fairly reliable heart rate for you.
Small handheld oximeters stop there. But larger models, such as the multi-purpose patient monitors used by medics and at hospital bedsides, will also display a waveform. This is a graphical display of the pulsatile flow, with time plotted on the horizontal axis and strength of the detected pulse on the vertical. With a strong, regular pulse, this waveform should be clear and regular, usually with peaked, jagged, or saw-tooth waves. Very small irregular waves, or a waveform with a great deal of artifact, is an indicator that the oximeter is getting a weak signal, and the calculated SpO2 (as well as the calculated pulse) may not be accurate. This waveform can also be used as a kind of “ghetto Doppler,” to help look for the presence of any pulsatile flow in extremities where pulses are not readily palpable. (To be technical, this waveform is known as a photoplethysmograph, or “pleth” for short, and potentially has other applications too– but we’ll leave it alone for now.)
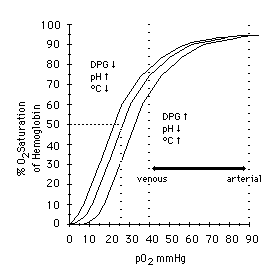
Most modern oximeters, properly functioning and calibrated, have an accuracy between 1% and 2% — call it 1.5% on average. However, their accuracy falls as the saturation falls, and it is generally felt that at saturations below 70% or so, the oximeter ceases to provide reliable readings. Since sats below 90% or so correspond to the “steep” portion of the oxyhemoglobin dissociation curve, where small PaO2 changes might correspond to large changes in SpO2 — in other words, an alarming change in oxygenation status — the fact that your oximeter is losing accuracy in the ranges where you most rely on it is something to keep in mind if using oximetry for continuous monitoring.
The lag time between a change in respiratory conditions (such as increasing supplemental O2 or changing the ventilatory rate) and fully registering this change on the oximeter is usually around 1 minute. And at any given time, the displayed SpO2 is a value calculated by averaging the signal over several seconds, so any near-instantaneous changes should be considered false readings.
Keep reading for our next installment, when we discuss the clinical application of oximetry, and understanding false readings.
Recent Comments