So let’s say we’ve stopped the bleeding as best we can. Now what?
The patient is still low on blood, and we know about all the problems this will cause. So shouldn’t we try and give them some back?
Well, maybe.
It makes sense that someone who loses blood should get some blood replaced. And this is a very old concept. Once upon a time, we simply drew blood from one person and gave it to another — a process that was greatly improved when we learned how to screen and test blood for compatibility and disease. This method is still used in some settings, such as the military, which treats its entire force as a “walking blood bank.” If Pvt. Joe needs blood, they check the registries to find a match, then call up Pvt. James and have him swing by to donate a few bags.
In most other settings, however, whole blood transfusion has largely become a thing of the past. Instead, when blood is donated, it’s immediately reduced to its constituent parts. The red blood cells are pulled out and stored as packed red blood cells (PRBCs); the platelets are pulled out and stored as condensed platelet concentrate; and everything that’s left — the plasma itself, including electrolyte-rich water, clotting factors, immune factors, and other ingredients — is frozen and stored as fresh frozen plasma (FFP). One unit of blood (around a pint) yields one unit of each component. Since most patients only need one or two of these components, we can divvy them out as indicated, and the same blood supply can benefit up to three people.
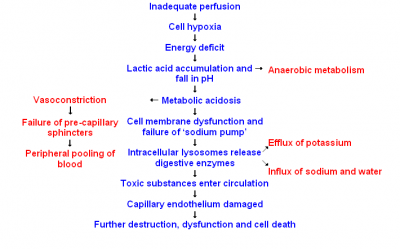
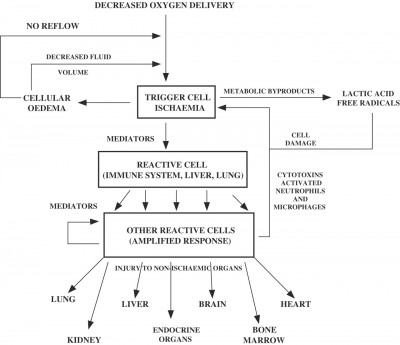
So for years it’s been standard to transfuse traumatic shock patients red blood cells. As we know, the key problem of shock is inadequate oxygen delivery, and red blood cells are how we deliver oxygen. So drop in a few extra hemoglobin, perhaps top them off with a bit of fluid to keep things moving, and we should be set, right?
Maybe. But this leaves out a number of factors.
First of all, remember our prime directive. Stopping the bleeding is more important than topping off the tanks. How does our body control bleeding? Platelet aggregation and coagulation. And remember that platelets, the bricks of this process, are not reusable; if we have a lot of trauma, and we lose a lot of blood, we can easily run out of them. Does transfusing red blood cells alone provide any platelets? Nope.
So maybe we should throw in some platelets too. But wait — we know that to actually bind the platelets into a cohesive clot, we need a host of backup players, the numerous coagulation factors that live in the plasma. Does a platelet pack provide these? Nope. (Okay, platelets are usually stored in a small amount of plasma, so there’s a few, but not enough.) So maybe we should give the patient some plasma too (or even isolated concentrates of clotting factors to really supercharge the process).
The result of all this is the recent movement towards so-called 1:1:1 therapy, where trauma patients receive equal proportions of red blood cells, plasma, and platelets. In other words, they end up getting all the individual components of whole blood; we just don’t often have whole blood available, or we might give that. This is still an area of active research, and the exact ideal ratios are up for debate; the ratio of red blood cells to plasma is often either 1:1 or very close to it (1:2, 1:3, etc.), and platelets are usually given in somewhat lower quantities, but should not be neglected. The best ratio, as well as the actual quantity of blood to ultimately give, remains to be seen.
Logistics can stand in the way of some of these efforts. For instance, plasma is typically stored frozen (as FFP), and therefore needs to be thawed before use, a process that takes some time. Very large trauma centers may be able to keep a rotating supply of thawed plasma on hand for emergency use, but many facilities won’t be able to have plasma immediately available in this way. And although transfusing in the field seems tempting, the practical challenges of carrying blood products on an ambulance are daunting.
Furthermore, banked blood is not “as good” as the patient’s own blood no matter how it’s given. Even a 1:1:1 transfusion, properly typed, screened, and cross-matched, has real risks of transmitting infection or causing an adverse reaction, carries less oxygen than fresh blood, has reduced hemoglobin pliability (the little disks “stiffen,” becoming less able to squeeze down capillaries to reach the hungry cells), and reduced numbers of labile clotting factors (particularly V and VII). It carries less 2,3-DPG, its pH is lower, and due to the anticoagulants and preservatives added for storage, it’s literally larger and more dilute than the whole blood it started as. Since transfusions are generally not our problem in the field, the applicable moral here is simply that “top ’em up” is not a simple or easy answer to shock, and the only intervention that truly keeps the patient out of trouble is to stop the bleeding!

From the Trauma Professional’s Blog at http://regionstraumapro.com/
In brief:
- Blood transfusion is an important step in treating traumatic shock, secondary only to controlling the source of hemorrhage.
- Modern “component” blood banking allows for the administration of almost any ratio of red blood cells, plasma, and platelets.
- Transfusing primarily red blood cells is the traditional approach, but a movement has recently developed toward more balanced ratios.
Next time: the legacy of crystalloids.

Recent Comments