Continued from Mastering BLS Ventilation: Introduction, then Mastering BLS Ventilation: Hardware, and finally Mastering BLS Ventilation: Core Techniques
We said before that robust management of the “A’s and B’s” requires having a wide range of options and tools available to you. At the BLS level, we don’t have many, but we do have a few. Now that we’ve explored the most important methods, let’s look at a few supplemental tricks and points to ponder.
Sellick’s Maneuver
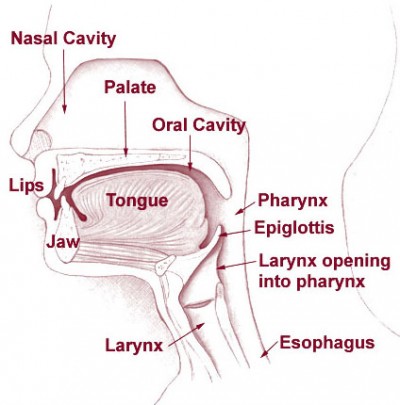
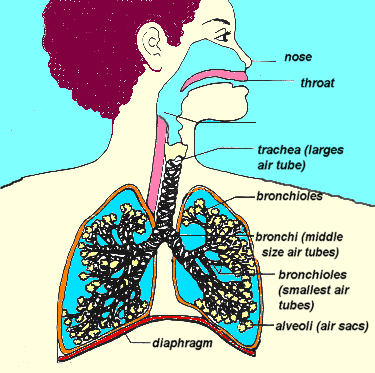
Once again, remember our upper airway anatomy: the larynx and trachea, through which air flows to the lungs, are positioned anterior to the esophagus, through which we’d prefer air did not flow. What’s more, these twin tubes are different types of structures. The trachea is built largely of cartilaginous rings, the same semi-rigid material that makes up the wobbly front of your nose; it’s not as stiff as bone, but it holds its shape well (go ahead, give your Adam’s apple a squeeze). The esophagus, on the other hand, is a fairly soft tube made of mostly muscle, and can easily be compressed flat.
This suggests a potentially useful trick. If we press upon the front of the larynx, it will retain its shape and move posteriorly, compressing the esophagus. In other words, although you’re pushing on the airway, it’ll remain open, while the esophagus behind it narrows and flattens. It’s like squishing a cardboard toilet paper roll with a metal pipe; they’re both tubes, but one is thin and easily distensible while the other is stiff and strong.
Since one of our challenges in BVM ventilation is getting air to go down the right tube, it makes intuitive sense that flattening the esophagus (the wrong tube) will help us push air into the trachea (the right tube). If we’re not successful with that, it may at least help prevent regurgitation from coming back out from the esophagus. This is particularly important because maneuvers like the sniffing position help straighten both of those tubes, so although they do open the airway, they also tend to increase the risk of gastric inflation. Worse, overly-aggressive bagging — from a first responder, for instance — can wedge open the LES guarding the stomach, and it can remain this way after you take over. Once someone’s forced it open, even gentle ventilations can enter the stomach.
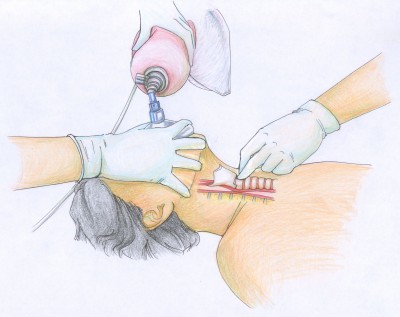
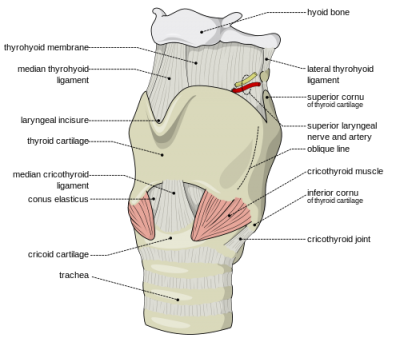
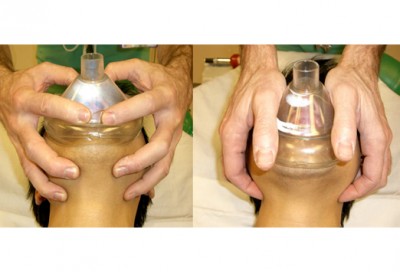
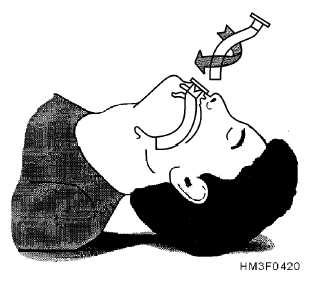
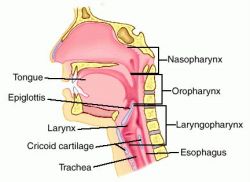
This is called Sellick’s maneuver, or simply cricoid pressure. It’s properly applied by pressing gently upon the cricoid cartilage, which is a good spot because the cartilaginous ring there creates a full circle (most of the other cartilages are C-shaped). It’s helpful during intubation, since it tends to move the glottic opening into the line of sight, but has also traditionally been used to assist with bagging.
To find the cricoid cartilage, palpate the most prominent bulge of the trachea, the “Adam’s apple” or laryngeal prominence. Move your finger downward over a small indentation (the cricothyroid ligament or membrane, where emergency cricothyrotomy would be performed) until you find another, smaller bulge. This is the cricoid cartilage.
Here’s the problem: theory aside, it often doesn’t work very well. A substantial body of evidence has shown that it often doesn’t do much to reduce gastric inflation, nor to impair regurgitation, and can even partially occlude the airway. This led the AHA to state that “. . . the routine use of cricoid pressure in adult cardiac arrest is not recommended” in the 2010 update to their BLS recommendations.
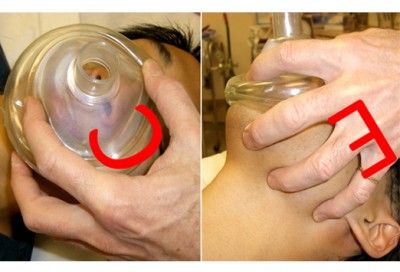
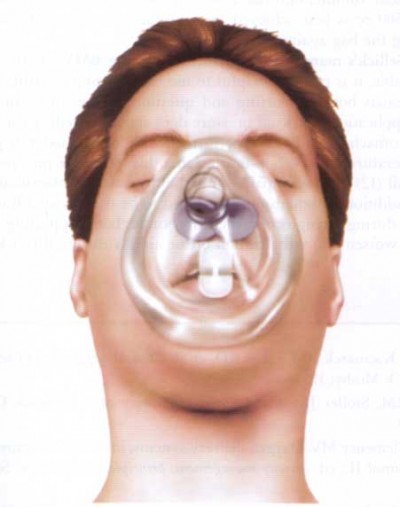
That doesn’t mean it’s useless, but it certainly suggests it shouldn’t be one of our first moves. It’ll help if we take care to do it correctly: pressure should generally be gentle (too hard and you’ll compress the semi-rigid larynx itself), straight back (it’s easy to “roll” to one side and fail to transmit the pressure to the esophagus), and applied nowhere but the cricoid cartilage. I also find that using your index and middle fingers, as in the illustration above, better facilitates this type of pressure than a thumb-and-forefinger grip. Use it as a last resort after other methods to minimize gastric inflation have failed — particularly the simplest and most effective, which is simply bagging with less force (ease the air in, don’t shoot it in) — titrate the amount of pressure to the desired effect, and in the end, don’t be surprised if it fails.
Pocket Masks
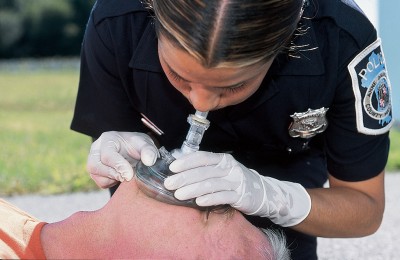
People may look at you like you’ve got six heads if you suggest it, but using a “pocket mask” is still a valid and indeed a recommended method for ventilation. Many BLS units carry the devices, which are essentially the same type of mask you see on the BVM, plus a port for supplemental O2 and a one-way or filtered valve to prevent cootie exchange. (If you don’t have such a device, you could simply detach the mask from your BVM and breathe into the hole, removing your mouth between breaths to let the patient exhale. This won’t be as effective of a barrier to infection, since there’s no one-way port, so it’s your call — but the risks are probably minor. You might even be able to increase FiO2 by leaving a cannula on the patient… or wearing one yourself.)
The advantages of this method are numerous. First of all, because you have two hands available to hold the mask, you’ll rarely have difficulty making a seal. Second, it’s extremely easy to titrate the volume and pressure of the breaths you give; unlike with the BVM, where you’re brusquely squeezing a rubber sac, with the pocket mask you’re using your pulmonary apparatus (your lungs) to assist the patient’s pulmonary apparatus, and it’s very easy to maintain tight control over the variables. Simply breathe in normally (not a deep breath) and exhale into the mask with gentle force, stopping when you see the chest rise. You should be able to do this with almost infinitely gentle pressure, making gastric inflation very unlikely.
The disadvantages: you can’t provide 100% oxygen, although if you attach the tubing and crank up a high flow, you can probably provide ample FiO2 for anybody without significant V/Q problems. But the bigger problem is the “ick” factor. Although research has shown that the risk of contracting an infectious disease during mouth-to-mask ventilation is very small, many providers still aren’t comfortable getting that close, preferring to literally stay at arm’s length. But remember: if you’re unable to effectively ventilate an apneic patient and you’ve exhausted all other options, this is a life-or-death situation, and ickiness should not be a key concern.
Mouth to Mouth
What if even the pocket mask fails, or for some reason you have no equipment of any kind available?
There’s always direct mouth-to-mouth ventilation. Nobody will fault you for opting out of this, because of the aforementioned ick factor and the theoretical chance of disease transmission, although again, research has suggested the risk is small. But if all else fails, it should be considered an option, and whether you’ll attempt it is solely up to you. Sheet-type barrier devices, which some people carry on their keychains, may reduce either ick factor or real risk, although you’re probably unlikely to find one around unless you carry your own. Remember that you’ll need to pinch or otherwise seal the nose; if your hands are busy maintaining an airway, you may be able to accomplish this by pressing your cheek against the nares.
If the mouth is obstructed or otherwise non-patent, mouth-to-nose ventilation is a viable alternative; simply ensure their mouth is shut and breathe into the nares. If a stoma is present in the neck, mouth-to-stoma or mask-to-stoma (an infant-size mask may yield the best seal) ventilation can be an option, although depending on how it’s constructed you may need to seal both the nose and mouth to make it work.
Just options, folks. Airways need options.
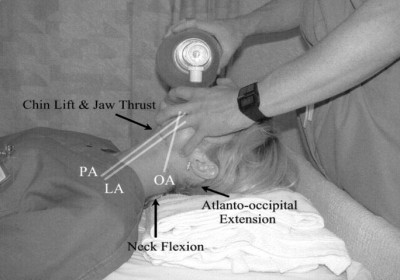
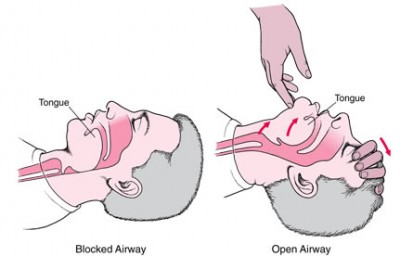
Jaw Thrusts
Along with manipulating the head, we know that shifting the jaw forward is essential for opening the upper airway. In fact, when we walked the Halls of the Student EMT, the wise men told us that for patients in spinal immobilization, it’s all we’re allowed to do. (A little later they usually said “. . . however, a patent airway takes priority over spinal precautions,” but most of us had already dozed off at that point.)
In any case, translating the jaw forward as far as possible, no matter how you do it, can open the airway substantially.
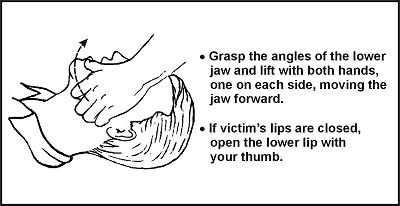
Along with the classic jaw thrust, there’s another method that’s rarely seen anymore. It’s real easy: with one hand, grab their mandible by the chin and lower teeth and pull up. It works. Could you get bitten? Yes. You also can’t bag them while you’re holding their jaw in your hand like Hamlet. So it’s more of a first aid tactic, but it’s very idiot-proof, so it’s nice to know about. You can see it working in this video.
Risk Factors for Difficult BVM Ventilation
It’s one thing to have a wide range of options for dealing with difficult-to-bag patients, but it’s also helpful to know before you dive in when a patient is likely to become difficult. It can help inform your decisions about priorities and flow of care, as well as the need for ALS and transport destinations.
Patients who are often challenging to bag include:
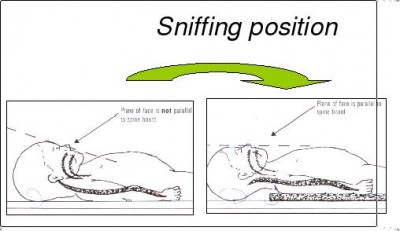
- The obese. Ample soft tissue tends to occlude the upper airway (this is why they often suffer from sleep apnea), adipose tissue bears down on their chest and diaphragm, and they’re generally difficult to position how you’d like. Ramp them and get a good sniffing position ahead of time (don’t try to dynamically head-tilt them while you apply the mask — situate them beforehand, so all you’ll need to do while you bag is maintain the jaw thrust), use airway adjuncts liberally, and plan ahead — don’t ever assume it’ll go smoothly, or you’ll find yourself in over your head without backup plans.
- Bearded patients. Thick beards and other facial hair make obtaining a mask seal difficult. It can help if you smear it down with some water-based lubricant (such as your NPA lube), but it can also make a mess of everything until you’re slip-sliding away like Paul Simon. You could also shave them a bit if you have a razor (with your AED gear, for instance), although they probably won’t thank you later unless it’s quite necessary.
- Sleep apnea. If you happen to know (via history) that the patient suffers from sleep apnea — or to a lesser extent, even that they snore at night — this indicates an existing predisposition toward upper airway occlusion when their level of consciousness is mildly depressed, so you can expect it to be that much worse when they’re entirely comatose.
- The elderly. Everything is harder with old people, including bag-mask ventilation, for numerous reasons.
- Anyone with a difficult-to-protract mandible. You probably won’t know this by looking, but if you go to initially address the airway and find that you’re unable to lift the jaw until the lower teeth are at least aligned with the upper teeth (preferably until they’re anterior), you’re probably going to have a hard time, and will need to compensate by achieving optimal extension and a sniffing position.
- Anyone with gross trauma to the face or neck, which may create airway occlusion, hinder your ability to make a mask seal, or generate substantial blood and other fluids requiring aggressive suctioning.
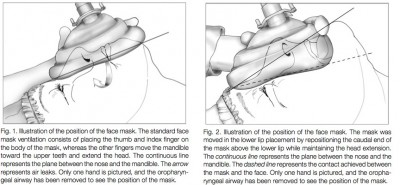
- Edentulous (toothless) patients. Aside from the fact that they’re usually elderly, patients without teeth have minimal structure to the oral cavity, giving you little to press against with the mask and obtain a seal. If dentures are present, it will help to leave them in; if not, make sure to place an OPA, which provides a little support at least. Make an effort to outwardly “spread” the air-filled skirt of the mask before applying it, which helps ensure that its maximum surface area remains in contact rather than curled uselessly underneath. Also consider this alternate mask placement, which may be more successful: the mask is shifted upward, so the lower edge meets the lower lip directly.
The End-Expiratory Pop
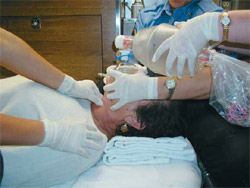
This is an interesting, unusual, and advanced technique which I’ve only ever seen advocated by the Department of Critical Care at the University of Pittsburgh. Briefly, it consists of the following: you bag with a two-person technique if at all possible, ensuring an excellent seal (which is mandatory) and letting you focus solely on the bag. You inflate as normal, release the bag and let the patient exhale, and then near the end of the expiratory phase, you “catch” them with a small squeeze to the bag, preventing their lungs from fully deflating. This may not seem possible, because there’s a valve present that allows exhaled air to vent, but that valve’s position is determined by the relative pressures on each side, so if you insufflate gas at a higher pressure than the patient’s exhaled gas, it’ll open in rather than out. This creates a sealed, temporarily closed system supported by the pressure you’ve created in the bag. If you don’t believe it, try bagging with the mask sealed against a table, or even upon your own face using clean gear.
View an example of the technique in this video clip, from :25 to :55. Here they’re simulating assisting with spontaneous respirations, probably one of the best applications for this method.
This yields two advantages: first, it gives you an excellent “feel” for pulmonary compliance. With a leak-free seal and balanced inspiration/expiration, compliance should remain consistent. If the resistance you feel suddenly decreases, you most likely have a leak. If it increases, you likely have either an obstruction or are “breath stacking,” failing to fully allow for expiration before beginning the next breath. With practice you can develop an excellent tactile sense of the bag-lung interface… as long as your mask seal remains flawless.
Second, and more profoundly, this actually creates positive end-expiratory pressure, or PEEP. In other words, you’re maintaining positive pressure in the lungs even after exhalation, where the alveoli ordinarily might collapse. By never quite “touching ground,” pressure-wise, you keep alveoli partially distended and portions of the bronchial tree “splinted” open that otherwise might have collapsed, particularly in disorders like COPD or CHF. This is the same principle used by CPAP or BiPAP devices, and it’s a wonderful boon that’s often the only way to effectively oxygenate patients with significant atelactasis (collapsed alveoli) and shunt (portions of the lungs that air is unable to reach). If you have a patent airway and are introducing adequate amounts of 100% oxygen, yet the patient remains hypoxic (according to skin signs or pulse oximetry), it’s almost certainly because of a V/Q mismatch like this, and that situation cannot be solved without PEEP or radically more aggressive measures.
The reason this trick is so cool is because it’s probably the only way to apply PEEP at the BLS level, since in most areas we do not carry CPAP devices, or even PEEP valves for the BVM. It’s theoretically possible to tape over or otherwise partially occlude the exhalation port of the BVM, narrowing the space for expiration and therefore providing some back-pressure, but this is totally unmeasurable, not easily titrated, and interferes with the entire phase of expiration. Although trickier, the “Pittsburgh PEEP pop” is better.
Why squeeze at the end of expiration? If you squeeze earlier, you’ll interfere with exhalation of gas, which needs to happen if we’re going to adequately blow off CO2 and avoid “stacking” breaths. If you squeeze later, you missed your chance to prevent a “zero pressure” state in the lungs, so you’re starting from zero again.
Key Points
- Sellick’s maneuver (i.e. cricoid pressure) can be helpful for reducing gastric inflation, but is often ineffective or even counterproductive. Use it as a last resort, applying only gentle and direct pressure, and if it’s not working, stop.
- Mouth-to-mask, mouth-to-mouth, mouth-to-nose, or mouth-to-stoma can all be effective backups to BVM ventilation, particularly when unable to achieve a mask seal or unable to ventilate without inflating the stomach.
- Expect obese, bearded, elderly, toothless, or traumatic patients to be difficult to bag.
- A small amount of PEEP can be created with a normal BVM using a small end-expiratory squeeze; this also helps confirm the ongoing integrity of the mask seal.
Next time we’ll give a method for combining all of these concepts into a cohesive approach to the BLS airway.
Continued at Mastering BLS Ventilation: Algorithms



Recent Comments