We brought up pulse oximetry several weeks ago, and it seems like a topic worth exploring in detail. What’s this device all about, and how should we be using it?
In order to get there, though, we should really start with some basics of pulmonology and respiration. Don’t worry — we’ll get to the good stuff soon enough.
Oxygen transport physiology
The cells of the human body use oxygen molecules (two oxygen atoms forming an O2) as a vital component of their basic metabolism. Most can survive briefly without oxygen, but not for long and not well.
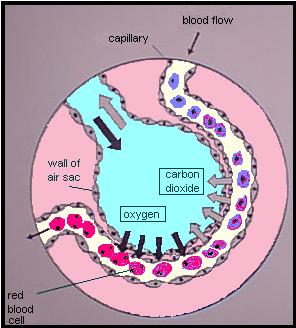
Delivering oxygen to the cells is a process that starts in the lungs. Oxygen in the ambient air is inhaled into the thin-walled sacs called aveoli, where they easily diffuse across the membrane wall into tiny capillaries filled with blood. (At the same time, carbon dioxide [CO2] is diffusing in the other direction, from the blood out into the alveoli, to be exhaled out as waste.) This oxygen “dissolves” into the blood in the same way that fizzy CO2 is dissolved in a can of Pepsi.
The concentration of oxygen present in arterial blood is a concentration called PaO2, and is directly related to the concentration of oxygen inhaled into the alveoli. (This is referred to as PO2, or the partial pressure of oxygen.) In other words, the more oxygen you breathe in, the more will cross over into the blood. Breathing faster and breathing higher concentrations of oxygen will both achieve this.
Just like in the Pepsi, the amount of oxygen your blood can dissolve is limited by the PO2 of the gas surrounding it. The trouble is that amount of oxygen you breathe in can only produce a very low PaO2 — nowhere near enough bloodborne oxygen to sustain human life. (The kinds of life that can survive on dissolved oxygen alone are the lumpy ones that just kind of roll around from place to place.) So animals like humans have developed a method of carrying far more oxygen in their blood than the fluid itself can absorb. We call it hemoglobin.
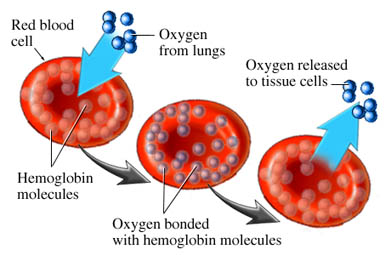
Hemoglobin are little iron-based proteins. We have zillions of them in our blood, and they like to cluster into donut-shaped discs called red blood cells (or erythrocytes).
Each hemoglobin has four binding sites where oxygen molecules like to attach. Each site can bind one oxygen, and only one. Four oxygens per hemoglobin is maximum occupancy.
So the process goes like this: We breathe oxygen into our lungs. It disperses across the thin membranes of the alveoli, entering the capillaries, where it dissolves into the bloodstream. This dissolved oxygen is then bound by circulating hemoglobin, like a fleet of buses. These drift downstream until they arrive at the tissue beds — muscle, skin, heart, liver, brain, anything and everything — where the process happens in reverse. The hemoglobin unload their oxygen, which diffuses across the cell walls, and is taken up by the cellular machinery for conversion into energy by aerobic metabolism.
Later, after the aerobic cycle has used up the oxygen, the waste fuel that comes from the other end will be carbon dioxide. This will diffuse back into the blood, where some is bound by hemoglobin, but the majority remains in solution (either unchanged or in the form of sodium bicarbonate); it returns to the lungs, reenters the alveoli, and is exhaled. The cycle is complete.
Oxygen delivery
This whole process is obviously critical. The delivery of oxygen from the lungs to the tissue beds requires adequate function of the lungs, of the blood itself, and of the surrounding environment that allows for oxygen binding and unloading.
In the lungs, this process can be compromised in numerous ways. As we saw, oxygen must enter the alveoli and blood must circulate through the alveolar walls in order for transfer to occur. These two processes are referred to as V (for ventilation) and Q (for perfusion). Inadequacy of either one is called a V/Q mismatch. For instance, obstructive lung diseases tend to decrease the total alveolar membrane available to oxygen — blood is still circulating there, but the gas can’t reach it. This is a failure of V (or shunt). A pulmonary embolism, on the other hand, blocks bloodflow to part of the lungs — you still breathe oxygen into those areas, but no blood is present to receive it. This is a failure of Q (or deadspace). (Obviously, someone who isn’t breathing at all will be inadequately oxygenated in a much simpler way.)
In the blood itself, other problems can occur. First, understand that the total amount of oxygen delivered to your body is not only determined by how much is bound to the hemoglobin, but also by how many hemoglobin are available. A low blood volume — such as in hypovolemia — will compromise this. A normal blood volume, but low hemoglobin count — as in anemia — will also compromise this. An adequate volume and hemoglobin count, but inadequate circulation — low blood pressure and poor cardiac output — will result in a “traffic jam,” with plenty of buses and plenty of passengers, but not enough movement from Point A to Point B.
There can also be problems with either the binding or unloading of oxygen.
The oxyhemoglobin dissociation curve
Adequate oxygen delivery depends on the hemoglobin binding, transporting, and ultimately unloading O2 molecules. As we saw, although oxygen does dissolve into the plasma itself, it is not nearly enough to sustain life; we need those hemoglobin working properly to act as ferries.
Each hemoglobin can bind zero oxygens, one, two, three, or four. How many it binds is directly related to how much oxygen is dissolved in the blood; the more oxygen in solution (PaO2), the more will bind onto hemoglobin (SaO2). If 50% of our total binding sites were occupied by oxygen (for instance, if all of our hemoglobin had two bound oxygen each), we would say our arterial blood is 50% “saturated” — an SaO2 of 50%.
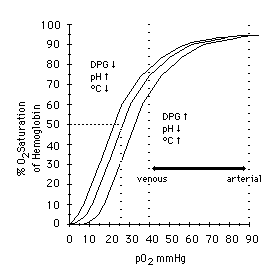
If we graph the PaO2 on one axis, against the SaO2 on the other, we get a line called the oxyhemoglobin dissociation curve. This describes what pressure of oxygen we need to achieve in the blood in order to reach a given saturation of hemoglobin.
Interestingly, this line will not be straight, but rather an S-shaped (or “sigmoid) curve. The reason is that although more oxygen means more binding, not all binding is the same. It takes a fair amount of pressure to bind the first oxygen, but once it’s bound, the affinity of that hemoglobin to bind is substantially increased. It now wants to bind more. Once it binds its second oxygen, its affinity is increased even more; it now takes very little additional PaO2 to bind at the third site. After the third, however, a certain amount of “overcrowding” comes into play, and the fourth binding site has a lower affinity than the third. The curve flattens back out.
Here’s the trick. This curve is not set in stone. It is determined by a number of physiological parameters, which can shift the line to the left or right.
Movement of the line to the right means that for a given PaO2, you will achieve less saturation. The affinity of hemoglobin for oxygen is low; it “doesn’t want” to bind, so you must reach a higher pressure of dissolved oxygen before it will attach to the hemoglobin. On the other hand, since it doesn’t want to be there in the first place, it will very readily unload at the tissue beds. Oxygen is hard to bind but easy to deliver. Factors that shift the curve to the right include: warmer temperatures; acidosis; and high 2,3-DPG (an “unload more oxygen” signaling molecule produced in hypoxic conditions, like COPD, CHF, airway obstructions, and high altitudes). These are all conditions seen in metabolically active states like exercise, where we need more oxygen down in the trenches.
Movement of the line to the left means that for a given PaO2, you will get more saturation. The affinity of hemoglobin for oxygen is high; it binds very readily, so little oxygen needs to be present before it will find a binding site. However, since the affinity between hemoglobin and oxygen is so strong, it will not want to unload into the tissues. It’s easy to bind but hard to deliver. Factors that shift the curve to the left include: cold temperatures; alkalosis; and low 2,3-DPG (of which inappropriately low levels are often seen in sepsis and iron deficiency).
Which do we want? Generally, moving the curve to the right is preferable in critical illness. Although it seems like a problem that we need to get more oxygen onboard, in reality this is usually possible with active medical intervention: we have supplemental oxygen, assisted ventilations, and at the end of the day can always just help someone do more breathing. However, what we can’t do is help them unload oxygen at their vital organs. For someone in a high-demand state, such as the shocked trauma patient, we want to maximize the delivery of oxygen to their body; the last thing we want is plump, well-saturated hemoglobin that refuse to unload their cargo where it’s needed.
Still awake? Tune in next time to hear about how oximetry works and what it should mean to you.
Keep reading with Pulse Oximetry: Basics and Pulse Oximetry: Application
fighting for air in many diseases is tough on your heart too! A major reason for fibrillation & Heart failure, I’m sure!
The amount of hemoglobin is a red blood cell depends on the P O2 of the plasma that surrounds the red blood cells and the avaliabilty of oxygen-binding sites within the red blood cells. The amount of hemoglobin increases with altitude.