Continued from Mastering BLS Ventilation: Introduction
The basic tool of BLS oxygenation is the bag-valve-mask, aka the bag-mask (as the AHA calls it), aka the Ambu-Bag (as most in-hospital staff call it, after one of the popular manufacturers), aka the self-inflating resuscitator. We’ll talk about techniques for optimizing for BVM success later. For the moment, let’s discuss some of the other auxiliary aids available. As we do, remember our main challenges: if we don’t minimize the resistance to airflow into the trachea, we’ll be prone to inflating the stomach instead of the lungs. And if we don’t minimize obstructions higher in the pharynx, we won’t be able to introduce any air at all.
Nasopharyngeal and Oropharyngeal airways
The NPA (or nasal trumpet) and OPA are the mainstays of BLS airway adjuncts. Essentially, they’re just curved pieces of plastic or rubber, designed to be inserted into the upper airway to prevent soft tissue from collapsing and obstructing the lumen.
When I first learned about these, it was just after hearing about the head-tilt chin-lift and jaw thrust, which were purportedly enough to open any self-obstructing airway. Why did we need these tools? “This way,” my instructor advised, “you don’t have to sit there holding their airway open.”
Well, yes and no.
The standard theory behind these devices is this: in a supine, unconscious patient, the tongue (and other soft tissue) wants to collapse into the pharynx. If we can jam something in the way, it will essentially “splint” open the passage — stick a foot in the door — much as if we were holding tissue back with a tongue depressor. Positioning the head and neck in such a way that it widens the relevant gaps would accomplish the same thing.
Under this thinking, we have several redundant tools to accomplish the same purpose. Whether we open the airway by tilting their head and lifting their jaw, or by sticking an OPA in the mouth, or by sticking an NPA in the nose, the result is the same.
But this doesn’t quite reflect reality. Sometimes it will, but in many patients with difficult airways, it’s not so simple to maintain a patent passage for airflow. In an obese patient with challenging upper airway anatomy, the amount of soft tissue standing in your way may be profound, and it can obstruct the lumen in multiple places. Additionally, tone may be so lacking that it easily “molds” around anything you stick in there.
In other words, if you place a BLS airway, the only breathable passage you’re really guaranteed is the lumen enclosed by the device itself: the central hole or grooves. And that’s not very much room. Our goal isn’t to create a tiny breathing tube, it’s to maximize the amount of usable airway — we’d like to be able to ventilate through as large a diameter as possible. That means using everything we can.
So proper positioning is helpful. So is an OPA. And perhaps an NPA. Or two.
In fact, if at all possible, it’s always worth trying to insert multiple airways. This is typically not taught to EMTs (since textbooks subscribe to the the “splinting” rather than the “protected lumen” theory), but it’s widely practiced in the ED and by experienced paramedics. If you’re having any difficulty at all bagging, shoot for an OPA with bilateral NPAs; filling all the available holes with patent airways is always a good idea.
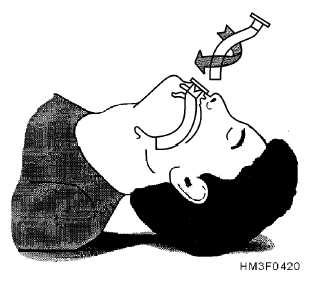
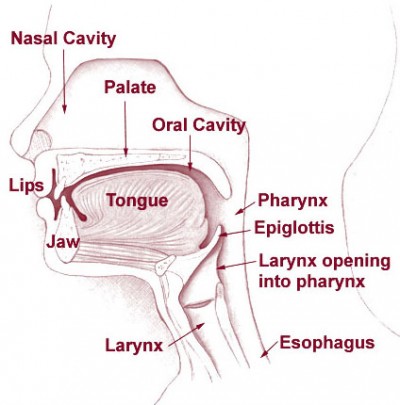
Remember what you’re actually doing with each airway. With an NPA, you’re separating the soft palate from the superior and posterior nasopharynx, and if it’s properly sized, it should be long enough to create a passage through the laryngopharynx, nearly to the epiglottis. (If it’s too long, it can stimulate the gag reflex, or jam into the vallecula or epiglottis, actually obstructing the larynx; if it’s too short, it may not protect the laryngopharnyx, or even may not fully span the nasopharynx, allowing the soft palate to shut.) With an OPA, you’re separating the lips, depressing the tongue to prevent it from obstructing the oral cavity, and more importantly protecting the laryngopharynx in the same way the NPA does — keeping the tongue or other anterior structures clear.
So if you only insert an NPA, the nose is your only guaranteed airway. If the mouth itself is shut — and we typically squeeze it shut when we bag using the “EC clamp” technique — nothing will flow through the oropharynx. Conversely, if we only insert an OPA, there is no guarantee that the nasopharynx will remain patent, particularly where the soft palate wants to meet the posterior pharynx.
So use both, because we want it all.
OPAs are more widely used, but it’s a shame to neglect the NPA. The advantage, of course, is that patients with an intact gag reflex can still tolerate an NPA, whereas the OPA may stimulate vomiting. It’s unwise to use the “try and see” approach with the OPA, because there’s nothing quite like copious emesis to make a difficult airway more difficult. Kyle David Bates teaches the helpful tip of inspecting for saliva and secretions collecting in the mouth; if there are none, the patient likely has an intact gag reflex. If they are present, an OPA is probably safe. But suction is always worth keeping on-hand and prepared.
It’s taught that NPAs are contraindicated in patients with significant facial or cranial trauma, on the theory that you may pass the device through a basal skull fracture right into the brain. This is probably a negligible risk; the entire concept seems to be based on two (yes, that’s the number before three) case reports in the literature. If your suspicion is quite high (blood from the nose with a positive halo test, for instance), you may want to steer clear, but with a truly difficult airway, remember that oxygenation is more important than an extremely remote risk of poking the patient’s noodle.
NPA placement can be facilitated by ensuring you lubricate the device first (water-based jelly should be available, although traditionally the patient’s saliva can be used as a last resort), aiming “in” (posteriorly) rather than “up” (superiorly), and lifting the nose to facilitate this angle. Also, remember that each nasal fossa has erectile tissue which takes turns engorging and partially obstructing airflow (allowing cyclical “resting” of the mucosa), so at any given time, one nare will likely allow easier NPA passage than the other; if you’re having difficulty, just switch sides. (Stripping part of this tissue away from the concha will occasionally cause post-insertion bleeding, but it’s rarely significant.)
As for the OPA, we usually teach insertion with the tip pointing up, followed by a 180-degree rotation once it’s fully inserted. Just remember that it’s also acceptable and sometimes easier to insert it tip-down while holding back the tongue with a tongue depressor or finger.
Another somewhat prosaic benefit to the OPA is that it may help provide structure to edentulous [toothless] patients when you’re trying to bag them, although simply leaving dentures in place can also work.
Apneic Oxygenation
You may not think that the lowly nasal cannula and non-rebreather mask really qualify as useful airway tools in an apneic patient. But oh, you would be wrong.
Pop quiz: is it possible to oxygenate the blood without actively moving any air? In other words, can you breathe without breathing?
You might say no. But why not? Gas exchange in the alveoli is not an active process; you’re not forcing the O2 molecules across the membrane by any chemical or muscular exertion. They simply diffuse passively, like gin dispersing into your tonic. All you’re doing when you breathe (either spontaneously or via positive-pressure ventilation) is providing a fresh supply of air to ensure that the concentration of oxygen in the alveoli remains higher than the concentration in the blood (thus allowing diffusion to occur). If we can keep the alveolar oxygen levels high without breathing, that’s just fine.
Suppose, for instance, that we place the apneic patient on a nasal cannula at relatively high flow. This should fill the pharynx with near-100% O2. Even without breathing, gas exchange is occurring in the alveoli; oxygen is diffusing across the membrane into the blood where it binds hemoglobin, and carbon dioxide is diffusing the opposite direction. Far less CO2 is moving out than oxygen is moving in, however (due to differences in solubility and hemoglobin affinity), so there’s actually a net “loss” of gas. This creates some “suction” or a partial vacuum in the alveoli, which will draw in whatever gas is waiting in the upper airway to fill it. Since we’ve flushed that space with pure O2, oxygen will move down that gradient, enter the alveoli, and continue diffusing into the blood, creating a continuous flow. Using this method, patients have been demonstrated to maintain reasonable sats for ridiculously long periods (up to 100 minutes in ideal circumstances).
This is a technique called apneic oxygenation. Although referred to by different names, it’s not new (among other things, it’s a traditional component of most brain-death evaluations), but it’s recently been getting more publicity. In particular, Scott Weingart of EMcrit and Richard Levitan recently published a paper comprehensively describing its use in difficult intubations. They advise placing a cannula at 15 L/min in order to suffuse the pharynx with near-100% O2, and this recommendation has some support in the literature. (Interestingly, whether the patient has their mouth open or closed may not matter.) We’re usually taught that nasal cannulae shouldn’t be used at flows this high, since it’ll dry and irritate the mucosa of the nose, and this is true; however, for short periods in critical patients, a dry nose is not the foremost concern.
How could this be useful for our purposes? Our main challenge with the BVM is ensuring that positive pressure goes where we want it to. This is obviously essential. But if bagging is initially challenging, could we potentially buy time? As long as the airway down to the glottis is open to flow, at least partially, it takes no skill at all to place a cannula (probably already present) and run up the flow to 15 L/min. Even if we’re totally unable to ventilate effectively, this will help keep the patient oxygenated and saturated while we work on a more definitive solution.
A couple of caveats: first, there must actually be a somewhat patent (if not totally secure) airway for this to work. If upper airway structures (or even a foreign body) have totally occluded the nasopharynx or laryngopharynx, no oxygen will reach the trachea. Second, this is a short-term temporizing measure only, because although it may help oxygenate, it will not help to “ventilate,” meaning to remove waste carbon dioxide; as discussed, CO2 is much less capable of passively diffusing without actual tidal movement to clear the alveolar space. Sustained apnea will therefore lead to continually increasing hypercapnia. Finally, this is really intended for patients with largely normal V/Q ratios; it will probably be of limited use for patients with significant shunt (e.g. bronchoconstriction, pulmonary edema, etc.) or dead space (e.g. pulmonary embolism). In other words, it’s of little help to your respiratory patients, whose problem is that their lungs aren’t working properly; if they’re moving air at all, they’re most likely suffusing their alveoli with high-concentration O2, it’s just that they’re just unable to exchange it. They need something like CPAP to help recruit more usable alveoli. Apneic oxygenation is for patients with working lungs who merely aren’t breathing spontaneously or adequately protecting their airway.
Can’t you just use a mask for this? Eh. Studies suggest that O2 from a non-rebreather tends to remain outside the face (in the bag and mask itself) unless the patient actually breathes, since it’s easier for the gas to simply overflow from the exhalation ports than to penetrate their airway; this is distinguished from the cannula, which actually shoots pressurized oxygen directly into the nasopharynx.
However, when it comes to patients who do still have some spontaneous respirations, a non-rebreather can certainly be useful, and here’s a way to supercharge it. Contrary to popular belief, you’re not actually delivering 100% oxygen with a typical mask at 15 L/min — more like 60–70% in most cases. This is due both to the poor seal it generally forms with the face and to the fact that at least one external port is usually left open to room air, so that if the oxygen supply is interrupted or becomes inadequate the patient won’t be suffocated. However, you can get closer to 100% FiO2 by simply cranking up the flow. Once you hit around 30–60 L/min, enough surplus oxygen is overflowing through the mask that the patient should be breathing nearly pure O2. Your portable oxygen tank probably won’t allow a flow this high (and it’d quickly run empty if it did), but most wall- or ambulance-mounted regulators should, although it may be near their maximum flood. Just crank the regulator up to 15 and keep turning until it won’t turn anymore; the indicator won’t change, but the flow will keep increasing. (Although I won’t be the one to recommend it due to the [likely overstated] safety concerns, you could probably also get good results by taping over any valveless ports in the mask, and holding it tightly sealed to their face — or better yet, letting them hold it.)
It may seem convenient, incidentally, to simply press a BVM against their face. Although this may — may — produce an effective seal, it provides poor O2 flow for spontaneous respirations; often times patient-initiated breaths simply bypass the reservoir and draw room air.
Key Points
- When it comes to BLS airway adjuncts, the more the better. Two NPAs and an OPA is ideal.
- NPAs are generally safe; the risk of penetrating the cranial vault is probably negligible.
- Don’t go poking around with the OPA in already-difficult airways; make an effort to determine whether a gag reflex is present before stimulating it.
- If an open airway to the lungs exists, but ventilations are difficult, a nasal cannula at 15 L/min is an excellent way to provide apneic oxygenation as a temporizing measure to maintain saturation.
- The only “high-flow” oxygen device on your ambulance for a spontaneously-breathing patient is a non-rebreather with flow of 30+ L/min.
A general reminder: although we are cavalier with failing to include in-line or footnoted citations, these are all evidence-based recommendations, and readers are encouraged to inquire for the literature behind anything that seems surprising or dubious.
Continued at Mastering BLS Ventilation: Core Techniques, then Mastering BLS Ventilation: Supplemental Methods, and finally Mastering BLS Ventilation: Algorithms
Our EMT book recommended looking for a blink reflex when lightly touching the eyelids. No blink = no gag reflex and it is safe to use an OPA.
This might work similarly – http://academiclifeinem.blogspot.com/2011/04/trick-of-trade-corneal-reflex-test.html. Drop a few drops of saline in their eye and see if they blink. No blink = no gag reflex.
I’ve heard that one, Greg, and as a general test of level of consciousness I’m sure it works — but I don’t know of any physiological reason why the correlation would be better than for other general stimulus tests. As far as I know it’s different reflex arcs and different cranial nerves. But I could be wrong — maybe there’s some research here.