
Sometimes, patients can’t breathe. When that happens, we need to breathe for them.
Simple enough. This is life support at its most fundamental, and many of the interventions classified as “BLS” are found here — techniques and devices for artificially supporting the body’s airway and breathing.
And it doesn’t seem so hard. When they taught it in class, it only took a day or two, and a few pages in the textbook encompassed the subject. How to size an OPA, how to hold the BVM, something about jaw thrusts, and you’re through. Spend a few minutes playing with a mannequin and now you’re an expert.
In the real world, though, this is not child’s play. Managing the airway of a sick, apneic patient is, at best, a high priority; at worst, it’s an unqualified catastrophe. Case reports and horror stories of airways gone wrong can be found under every roof: the failed intubation, the disastrous cricothyrotomy, the foreign body obstruction that couldn’t be cleared. These are emergencies because as we all know, without an airway, you cannot survive. It’s simple stuff.
And then there’s the BVM — aka the bag-valve-mask or “Ambubag.” Ask a room full of novice EMTs and they’ll all agree it’s about as straightforward as tying your shoes: slap it on, squeeze, any idiot could do it. But ask the senior medic in the corner, and he may paint a grimmer picture. Jeff Guy has described it as a more difficult skill than endotracheal intubation, yet one of the hot topics today in prehospital medicine is whether paramedics should remove intubation from their scope of practice because it’s too hard. But nobody’s going to take away the BVM. It’s irreplaceable; it’s the first and last line, the means of ventilation that any patient starts with, and the fallback if your next move fails. The only problem is that doing it well, and for really tough patients, doing it at all, is a purely skill-based exercise. It’s the Jedi’s lightsaber: simple, versatile, but designed for an expert.
The point is that establishing a patent airway in a sick person who can’t do it themselves, and ventilating them using that airway, is such an important task that it generally mandates a large toolbox. Airways are often managed via complex flowcharts or algorithms, where one method can yield to another if it fails, and then to another and another. Countless different devices and methods are available, so that even when obstacles are present, any moron can stumble onto something that works before the patient crashes altogether.
And then there’s us. The Basic EMT stands at the bottom of the spectrum in terms of training, yet is expected to oxygenate any patient using nothing but the meager BLS jump-kit. He has the BVM, a couple of basic airways, masks, cannulas, suction, positioning — and beyond that, just his wits and skills. And as for those, he probably spent little to no time actually practicing them in class, and may perform them only rarely in the field.
This won’t do. When it comes to psychomotor skills, these are the most essential, because we don’t have a Plan B. If BLS techniques fail, our only recourse is to sprint for the hospital or ALS, and hope nobody dies along the way.
So let’s talk about all the principles and tricks of creating a BLS airway and ventilating with the BVM. First, we’ll need to understand why it’s hard.
Basic Physiology
Ordinarily, we suck at breathing.
I mean we literally suck. We drop the diaphragm and widen the ribs, expanding the area inside our chest. This expands the lungs, forcing them to suck air into the only opening available — through the mouth and nose, down the pharynx, through the trachea, and into the bronchial tree.
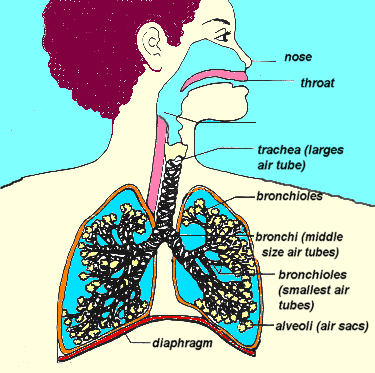
That’s assuming that the airway is open, of course.
Now, what if I whack you over the head, and your body loses the ability to spontaneously breathe? We’ll want to breathe for you. Can we pull down your diaphragm and expand your chest? Not very easily, unless we stick a plunger on your sternum, or put you in an iron lung. Instead, we reverse this process: rather than creating negative pressure inside the chest, we force positive pressure in from the outside. Rather than sucking, we blow.
Blowing is a little tricky, though. One of the main problems is that there’s more than one place for air to go. Consider the pharynx, the working area of your upper airway. We can get there via two paths: the oropharynx (via the mouth and over the tongue), or the nasopharynx (via the nostrils), but they arrive at the same place, the laryngopharynx (or hypopharynx). What happens next?
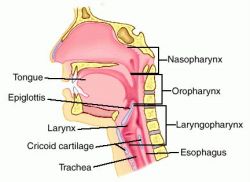
If we peered into your hypopharyngeal space, we would see that two openings emerge below. One leads to a tube which lies posterior (toward your back): your esophagus, which conveys cheeseburgers and beer into your stomach. One leads to a tube which lies anterior (toward your front): your trachea, which brings air into the lungs for gas exchange. Remember these relative positions — the trachea is in front, and you can palpate it at the neck (the “Adam’s apple” is part of it). The esophagus lies behind this, and is not usually externally palpable.
Given that food and air both enter via the pharynx, how do we ensure that cheeseburgers ends up in the esophagus and air ends up in the trachea? Well, the gatehouse to the trachea is the larynx (the “voicebox,” where vocalization occurs), and the opening to this chamber is called the glottis. The glottis is normally open, but when you swallow, a couple of drape-like vestibular folds and a little flap, the epiglottis, are pulled in to cover the larynx. The result is that food is forced into the esophagus.
What about the other direction? The esophagus is formed from rings of muscles called esophageal sphincters, which help “milk” food downward when you swallow. The bottommost ring is the lower esophageal sphincter, which opens during swallowing, but otherwise is mostly constricted, sealing off the esophagus from the stomach itself. This prevents air from passing down and gastric contents from coming up (something we know as heartburn).
To summarize, as you sit here reading this, your esophagus is clamped off by your lower esophageal sphincter, and your trachea is open, allowing you to breathe. But if you take a bite of your coffee-cake, your epiglottis and vestibular folds will block off your airway, your esophageal sphincter will open, and the food bolus will be directed into your stomach.
Down the wrong pipe
The trouble with blowing instead of sucking is that we have no way of aiming where we blow.
I know what you’re thinking. If we force air down the pharynx, the esophageal sphincter should block off the stomach, ensuring that it flows into the larynx and down the trachea. Right?
Here’s the problem. Even ordinarily, your esophageal sphincter only clamps down with a small amount of force — say around 30 cmH2O (centimeters of water, a unit of pressure). This is plenty to prevent air from flowing in during regular respiration. But if air were to be pushed in with greater than 30 cmH2O of force, it will squeeze past the sphincter and enter the stomach. And if we clamp a BVM over your face and squish the bag, we can easily exceed that much pressure.
It gets worse. In order for the esophageal sphincter to work even that well, it requires muscular tone (constant stimulation), just like your postural muscles need tone to keep you from falling over. What happens when you’re unconscious? Sphincter tone decreases. So in the people we’ll actually be bagging, opening pressure may be 20–25 cmH2O or even less. Thus it’s even easier for positive pressure ventilations to force their way into the stomach.
The result? When squeezing the BVM, air often enters the stomach along with (or instead of) entering the lungs. Not only is this pointless, it makes it even harder to inflate the lungs (a bigger abdomen creates pressure on the diaphragm), decreases cardiac preload, and increases the risk of vomiting — which will further obstruct the airway.
The easiest solution is to put a tube into the trachea and seal it off — i.e. endotracheal intubation (or variations on that theme, such as a blind airway). Then we can blow air directly into the lungs without any chance that it’ll enter the wrong pipe. Unfortunately, those are tools we often lack as BLS providers.
Angles and Tissues
All of those structures we’ve been describing? They’re soft.
Soft and squishy. And it’s not just the esophageal sphincter that loses tone when you become unconscious.
In ordinary circumstances, the airway is a supple but structured arrangement of tissues that maintains its form. This is important, because there’s not very much space in there. So in the unresponsive patient, it’s no surprise that some of those tissues might collapse together, blocking off the lumen between them. (Check out this fluoroscopic video.)
The tongue is the worst. Tongues are basically big blobby muscles, attached at only one end, and if you remove all firming tone, they just flop wherever gravity takes them. So put an unconscious person supine, and gravity pulls the tongue back into the pharynx, blocking all airflow.
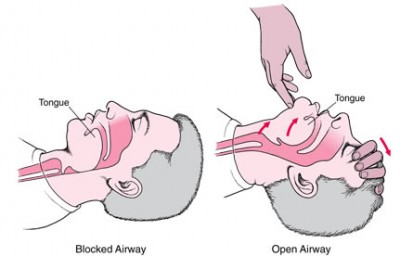
Or the larynx and supralaryngeal tissues run into the posterior pharyngeal wall. Or the soft palate does. Either way, anterior structures end up touching posterior structures, leaving no room in between. Our airway involves a tight 90 degree turn, and this is not a design that remains open without active maintenance. So if we want to breathe for these people, we need to find a way to unblock everything. (Like the jaw thrust — check out this airway cam.)
Mask Madness
Trying to push air into someone’s lungs by holding a mask over their face is like trying to blow up a tire by… well, holding a mask over the valve.
I teach CPR, and I can count on one hand the number of times I’ve handed the BVM to somebody and watched them achieve chest rise on the mannequin the first time. Heck, I demo the things and I don’t always pull it off.
Effectively sealing an air-filled plastic mask to someone’s face and then squeezing the bag is a task meant for more hands than any human possesses. Doing it on somebody who’s dying is exponentially more difficult. Add in the fact that they’re probably obese, toothless, vomiting, crumpled in a corner or bouncing around an ambulance, and enshrouded in a thick ZZ Top beard. Now try to get it all done without losing your cool or breaking your proper ventilatory rate. Having fun yet?
Key points
- BLS ventilation using basic airways, positioning, and the BVM is a difficult, complex, and undertrained skillset for the EMT-B. Yet since we often lack rescue devices or alternate ventilation methods, it is critical that we learn to master it.
- Preventing gastric inflation would be difficult even in healthy people, and is extremely difficult in the apneic and unresponsive patient.
- Loss of tone in unconscious patients lying supine reliably produces soft tissue airway obstruction which must be cleared.
- Obtaining a proper mask seal is a necessary prerequisite for BVM use, but is often difficult or impossible for a single rescuer.
Tune in next time to see some solutions to these challenges.
Continued at Mastering BLS Ventilation: Hardware, then Mastering BLS Ventilation: Core Techniques, then Mastering BLS Ventilation: Supplemental Methods, then finally Mastering BLS Ventilation: Algorithms


Recent Comments