Being reluctant to force Joe into an undesired ambulance ride, the crew contacted their supervisor. He arrived, evaluated the patient, agreed with their conclusions, and called Dr. Scrubs to discuss the matter. He was unable to dissuade the doctor from his decision.
The crew and supervisor approached Joe together and informed him of the circumstances; although all parties agreed that he should rightly be able to refuse transport, they felt they had been overruled by a higher authority, and if he would not come voluntarily they would be forced to compel him. Under this duress, Joe finally agreed to be transported, loudly and vocally protesting.
He was taken to his preferred hospital and care was handed off to staff with a full description of the situation. Less than 30 minutes later, another crew was sent back to the hospital to return Joe home; the attending ED physician had deemed his involuntary hold to be invalid and inappropriate, and refused to hold him against his will. No further evaluation was performed.
The encounter was documented extensively and quality improvement measures involving EMS and the base physician are expected.
Discussion
This case was not medically complicated, but it involved some difficult issues of consent and risk. Let’s look at the medicine and then at the wrinkles.
Medical Considerations
We were dispatched for a chief complaint of a fall — a very common mechanism of injury. When evaluating the fall, what should our main concerns be?
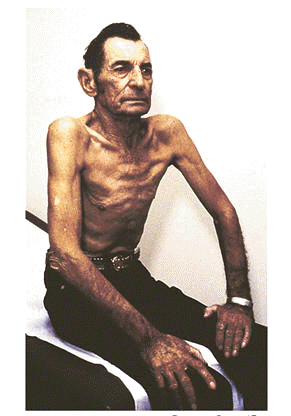
First, we should examine the mechanism itself. How far was the fall? In this case, as it often is, the fall was from a standing height, and from a standstill (i.e. not propelled while running, stumbling while breakdancing, etc.). This is often seen as the dividing line for significant versus non-significant falls; in many areas, falls from standing height or greater are considered an indication for spinal immobilization. (Other areas say greater than standing height; 3x standing height or more; or other numbers.) The elderly in particular are considered at higher risk for spinal injury, due to weakened bones and tighter ligamentous connections between vertebrae.
Typically, a blow to the head with loss of consciousness is also considered high risk for spinal injury. This is under the assumption that a blow with enough force to cause LOC may also have enough force to damage the spine. These considerations are all valid, but should only be seen as some of the many factors involved in stratifying risk; they must be considered alongside other elements like the physical assessment. In some systems, you may be forced to immobilize based on mechanism without other considerations. In others, you may be allowed to rule out immobilization based on certain findings, most of which Joe has; for instance, he denies neck or back pain or tenderness, denies peripheral parasthesias (numbness or tingling) or weakness, ambulated well, turns his head, and has no confounding factors like a distracting injury or altered mental status. In any case, the post-fall presentation was so benign that risk seemed low, and given the patient’s overall reluctance it is highly unlikely that he would have consented to a collar and board.
The use of warfarin (trade name Coumadin), on the other hand, does significantly increase the risk of intracranial hemorrhage (ICH), especially after blunt trauma to the head. Although again, Joe’s assessment was very reassuring — normal vitals, no complaints, and a baseline neurological status — it is very possible for ICH to have a delayed onset of presentation. The best example of this is the subdural hematoma, where cases of moderate severity sometimes take hours or days to develop, due to the venous rather than arterial source of bleeding. This delay is particularly common in the elderly, where (possibly due to shrinking of the gray matter, which leaves additional room for blood to collect before pressure begins compressing the brain) a classic scenario is the fall with a blow to the head, no complaints for hours afterward, and then sudden deterioration. Some sources state that 60% of geriatric fall patients who experience LOC from a blow to the head will eventually die as a result. Since in this case, we were delayed on scene for quite some time, there would be value in ongoing and repeated assessments of symptoms, neurological status, and vital signs while we waited around.
The patient’s pupils were unusual in appearance, which can be an indicator of brain herniation; however, this syndrome typically presents with one very large and round pupil. An irregularly shaped pupil as we saw here is more indicative of a structural defect, the most common of which is probably cataract surgery, which can leave the pupil off-round.
An incomplete medical history is common in scene calls involving the elderly. However, many do carry med lists, and in most cases you can reconstruct the majority of the patient’s diagnoses based on their medications. In this case, we found digoxin (or digitalis), which is almost always used to control atrial fibrillation; this is consistent with the patient’s irregular pulse, and with the warfarin, which helps prevent A-fib induced clots. Metformin (Glucophage) is an antidiabetic that helps control glucose levels. Citalopram (Celexa) is a common antidepressant of the SSRI type. Advair (fluticasone and salmeterol) is a preventative asthma/COPD inhaler combining a steroid with a long-acting beta agonist; it is used regularly to minimize flare-ups and is not a rescue inhaler. Omeprazole (Prilosec) is used for gastroesophageal reflux disease (GERD), aka heartburn. Ibuprofen is a non-steroidal anti-inflammatory (NSAID) used for pain relief and reduction of inflammation.
As VinceD noted in the comments, one essential question in any fall — and indeed in almost any traumatic event — is what caused it. Here we have a somewhat vague account which suggests a mechanical fall, i.e. tripping or loss of balance; this is not necessarily benign, as a history of repeated mechanical falls suggests deteriorating coordination or strength, but it is usually not indicative of an acute medical problem. However, many elderly patients (and some of the younger ones, too) will attribute any fall to tripping, so this claim should be taken with a grain of salt. It helps to have a witness to the event, as we do here, although witnesses are not always reliable either. In any case, what we want to know is: what happened just before the fall? Was the patient simply walking and tripped on a rug? Did he have seizure-like activity? Was he standing normally when he suddenly lost muscle tone and collapsed? Did he complain of feeling faint or dizzy? Was he exerting himself or straining on the toilet? Things happen for a reason.
Ethical and Legal Considerations
The bigger question is whether it’s okay for Joe to refuse transportation.
This is an odd question, because ordinarily we assume that people are free to go where they want, and calling 911 (or having it called for them) does not surrender this right. However, there is an attitude among those with a duty to act, such as healthcare providers and public safety officers, that individuals who are not cognitively able to understand their situation and make decisions in their best interest need to be protected from their own impaired judgment. This is equivalent to taking your friend’s keys so he won’t drive drunk, under the assumption that he wouldn’t want to drive drunk were he making sensible decisions. The legal term is implied consent, the same principle by which we transport children, drunks, and unconscious people.
How do we know if somebody is unable to make their own decisions? There is not an obvious line. For many providers, their rule of thumb is the old “A&Ox4”: if someone knows who they are, where they are, when it is, and what’s going on, then they are alert and oriented and capable of making decisions. Of course, this is only one piece of the mental puzzle. Social workers, psychiatrists, and other specialists have a full battery of tests that can help further reveal cognitive capacity. Can you perform these in the field? It’s probably more than you’re likely to do, although you might perform something simple like the MMSE. But some basic questions that highlight the patient’s judgment can help supplement your routine assessment — questions like, “Suppose you were at the mall when you started to smell smoke and heard the fire alarm. What would you do?” where any rational response is acceptable.
It’s important for the patient to be able to demonstrate that they understand what’s going on. Even someone with ordinary mental competence — unless they’re a fellow knowledgable healthcare professional — needs to be informed (to the best ability of the provider) of the possible risks and consequences of refusing care. In this case, it would involve giving them some description of the above possibilities (spinal fracture, head bleed, etc.), and ideally having the patient then relate them back to you, demonstrating good comprehension of those facts. The base physician’s view that Joe hadn’t fully demonstrated this understanding was a key part of his decision that he needed to be transported against his will.
Other important points are to ensure that the patient knows that refusal doesn’t preclude future care (“if you change your mind, you can always call back”); and that the ability of the providers to evaluate the patient on scene is at best limited. Any implication that you know what’s really happening to the patient or can definitively rule in or rule out any medical problem is unwise and legally risky. In fact, even suggesting possibilities or probabilities can be problematic if you’re wrong; on the other hand, failing to do so can leave them uninformed, so this can be a Catch 22. Your best bet is to outline some basic possibilities, carefully inform them of the limits of your training and resources, and be smart enough that you generally know what you’re talking about in the first place.
One complication in this case is the presence of someone who claims to be Joe’s health care proxy. A proxy (closely linked to the idea of a durable power of attorney) is a person whom, while of sound mind, you designate to make decisions for you if at a later time you are not of sound mind. Crucially, if you are still capable of decision-making, a proxy does not have the ability to override you; their role is to act on your behalf when you cannot. In other words, the decision of Joe’s proxy is only relevant if we do find (or in some areas, if an authority such as a judge has decided) that he’s incompetent to refuse or consent to treatment; thus, her presence does not necessarily alter the basic dilemma.
In this case, the physician’s attitude was that the problem was primarily medical: does the patient need emergency department evaluation to rule out dangerous processes? Medically, he does. However, the first question actually needs to be: Is the patient capable of evaluating risk and making decisions in his own best interest? If he is, then he is technically “allowed” to decide whatever he wants. Even a clearly dying man can refuse medical care based on religious views, personal preference, or any reason whatsoever (although barring a proxy or advanced directive, once he’s unconscious he can usually be treated under implied consent). This is different from the person who actively tries to take his own life; for philosophical reasons we view this as different from passively allowing oneself to die for lack of medical treatment. We prevent people from committing suicide but allow them to refuse medical care.
Realistically, although this fundamental right does not change, it’s fair to consider the surrounding medical circumstances to help decide how pressing and high-risk the matter is. In this case the doctor clearly felt that the risk was so high that it required going to extraordinary lengths, including overruling the patient’s own decisions and potentially even harming him, to ensure that a dangerous situation wasn’t “missed” — in short, that the ends justified the means. Dr. House is famous for this approach.
Legally, in most areas EMS providers are seen as operating under the bailiwick and legal authority of their medical director, and online medical control is an extension of this authority. In other words, within reason we are bound by the orders of medical control. The details of this relationship vary, and are not always fully explored. For an example, consider this true story from 1997 in New Jersey:
A North Bergen dual-medic crew is dispatched to a pregnant, full term female in cardiac arrest. Downtime is unknown, and they work the code for a number of minutes without response. Determining that the mother is likely unsalvageable, and concerned for the health of the fetus, they contact medical control. After a “joint decision” the base physician verbally talks them through performing an emergency C-section on scene. They deliver and successfully resuscitate the fetus, and both patients are transported. The mother is declared dead soon afterwards, but the infant lives for a number of days before dying in the hospital. In the aftermath, the paramedics are cited for violating their scope of practice, and their licenses to practice are revoked in the state of New Jersey. The physician is forced to undergo remediation training to maintain his medical control privileges.
Is the moral that acting in the patient’s best interest is not always a defense against liability? Maybe. Is the moral that medical control cannot authorize you to perform otherwise illegal acts? Maybe. Is the moral that we should protect ourselves before the patient? I don’t know about that, but it’s something to think about. In this case, the course for Joe that seems most ethical to me — allowing the patient to make his own decisions — also lets us avoid potential liability for battering and kidnapping. However, it does force us to refuse a direct order from medical control. Invoking our supervisor gives us a bigger boat either way, and would be a big help to protect us from trouble coming from our employer, one of the most likely sources. It’s also true that, while we may have believed that Joe was competent, he is at least somewhat diminished, so we’re less than completely confident. Nobody wants to put themselves on the line by taking a stand, only to be proven wrong.
Fortunately in this case we were able to avoid getting violent at all, but it was a near thing. If it did prove necessary, it should have been done with ample manpower and many hands; in some areas chemical sedation by paramedics may also be authorized. And I would certainly not recommend acting without the doctor’s signature on a legal document.
With everything viewed in retrospect, the situation would have been much more easily resolved had the doctor not been involved in the process. At the same time, however, if a simple refusal had been accepted, and CQI later went over the call — especially if Joe experienced a bad outcome — the crew would have been in a difficult place.
No matter what, such a situation is highly unusual, flush with liability, and should be thoroughly documented in all respects.

Recent Comments